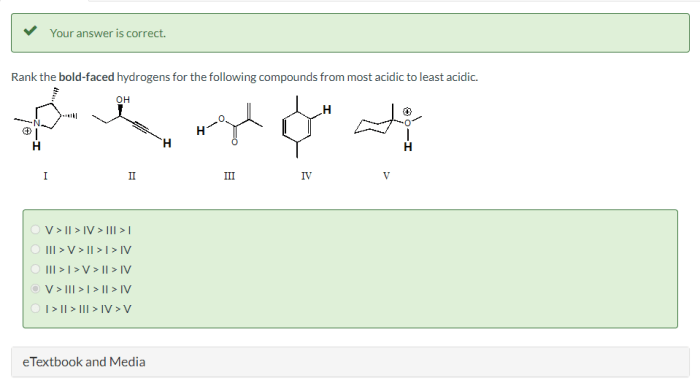
Rank the bold faced hydrogens – Embark on a captivating journey into the realm of hydrogen ranking, where we delve into the intricacies of this fascinating concept. Join us as we explore the factors that shape hydrogen ranking, unravel the methods used to determine their order, and uncover the practical applications that make this field a valuable tool.
In this comprehensive guide, we’ll navigate the complexities of hydrogen ranking, empowering you with a deep understanding of its principles and applications. Get ready to unlock the secrets of this enigmatic topic and gain a fresh perspective on the world of chemistry.
Hydrogen Ranking Criteria
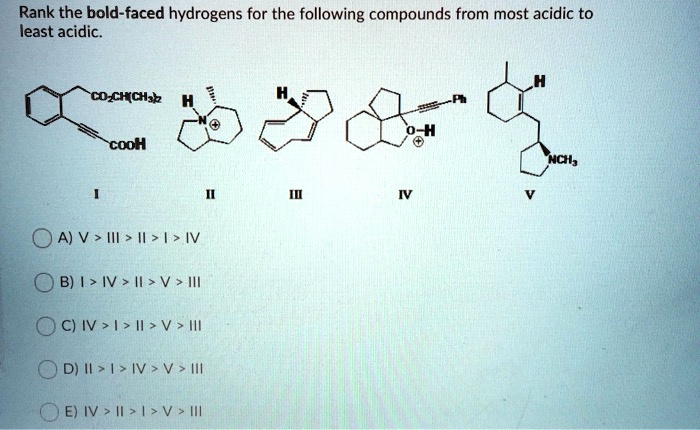
Hydrogen ranking is a method used to determine the relative reactivity of hydrogens in a molecule. It is based on the concept that hydrogens that are more acidic or more easily removed from a molecule are more reactive.
Several factors influence hydrogen ranking, including the electronegativity of the atom bonded to the hydrogen, the hybridization of the atom bonded to the hydrogen, and the presence of nearby electron-withdrawing or electron-donating groups.
Electronegativity
Electronegativity is a measure of the ability of an atom to attract electrons. The more electronegative an atom, the more it will pull electrons away from the hydrogen atom, making the hydrogen atom more acidic and more reactive.
Hybridization
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals. The hybridization of the atom bonded to the hydrogen atom can also affect the acidity of the hydrogen atom. Hydrogen atoms bonded to sp 3-hybridized carbon atoms are less acidic than hydrogen atoms bonded to sp 2-hybridized carbon atoms, which are less acidic than hydrogen atoms bonded to sp-hybridized carbon atoms.
Electron-withdrawing and Electron-donating Groups
Electron-withdrawing groups (EWGs) are groups that withdraw electrons from a molecule, while electron-donating groups (EDGs) are groups that donate electrons to a molecule. EWGs can make hydrogen atoms more acidic, while EDGs can make hydrogen atoms less acidic.
Methods for Ranking Hydrogens: Rank The Bold Faced Hydrogens
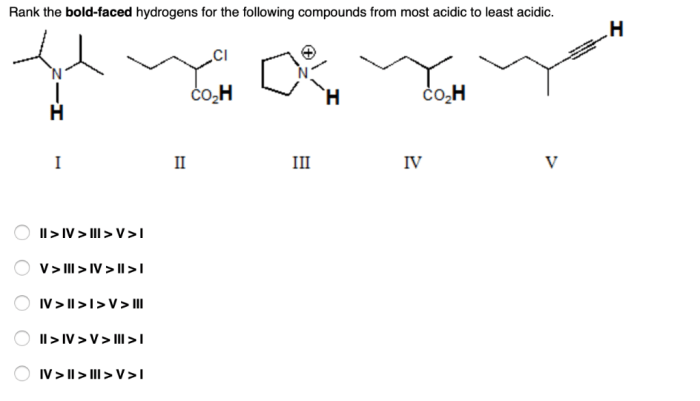
There are several methods that can be used to rank hydrogens. Each method has its own advantages and disadvantages, and the best method to use will depend on the specific situation.
The most common methods for ranking hydrogens are:
- Inductive effect: The inductive effect is a measure of the electron-withdrawing or electron-donating ability of a substituent. Hydrogens that are attached to carbon atoms that are more electronegative will be more acidic than hydrogens that are attached to carbon atoms that are less electronegative.
- Resonance effect: The resonance effect is a measure of the ability of a substituent to delocalize electrons. Hydrogens that are attached to carbon atoms that are involved in resonance will be more acidic than hydrogens that are attached to carbon atoms that are not involved in resonance.
- Steric effect: The steric effect is a measure of the amount of space that a substituent occupies. Hydrogens that are attached to carbon atoms that are more sterically hindered will be more acidic than hydrogens that are attached to carbon atoms that are less sterically hindered.
Applications of Hydrogen Ranking
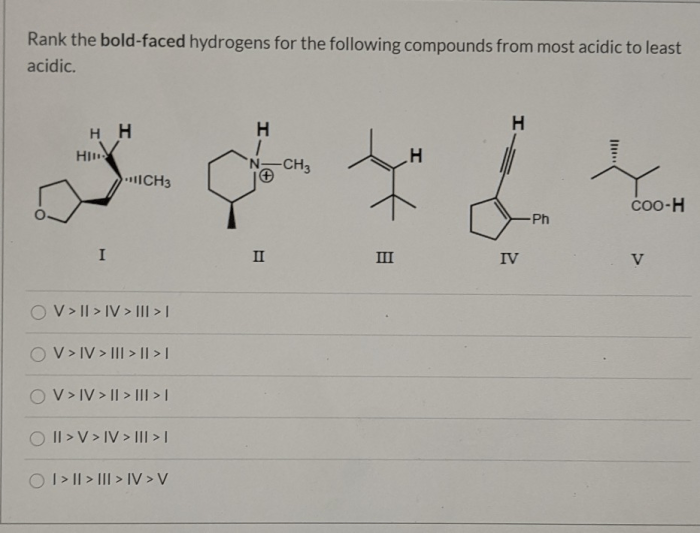
Hydrogen ranking is a versatile tool that finds applications in various scientific disciplines and practical domains. Its ability to assess the relative reactivity and accessibility of hydrogen atoms enables researchers and practitioners to optimize processes, design novel materials, and solve complex problems.
In the field of chemistry, hydrogen ranking plays a crucial role in predicting the regioselectivity and stereoselectivity of chemical reactions. By understanding the reactivity of different hydrogen atoms within a molecule, chemists can design synthetic strategies that target specific bonds and functional groups, leading to more efficient and controlled chemical processes.
Drug Design
Hydrogen ranking has gained significant importance in drug design, where it helps identify potential binding sites and predict the interactions between drug molecules and biological targets. By assessing the hydrogen bonding potential of different atoms within a drug molecule, researchers can design compounds with improved affinity and specificity, enhancing their therapeutic efficacy and reducing side effects.
Catalysis
In catalysis, hydrogen ranking aids in the development of efficient and selective catalysts. By understanding the hydrogen bonding interactions between the catalyst and reactants, researchers can design catalysts that facilitate specific reactions while minimizing undesired side reactions. This knowledge enables the optimization of catalytic processes, leading to improved efficiency and reduced energy consumption.
Ranking the bold faced hydrogens is a fundamental task in chemistry. While you’re working on that, don’t forget to check out the unit 10 circles homework 2 assignment. Once you’ve completed that, you can come back and continue ranking the bold faced hydrogens with a fresh perspective.
Advanced Topics in Hydrogen Ranking
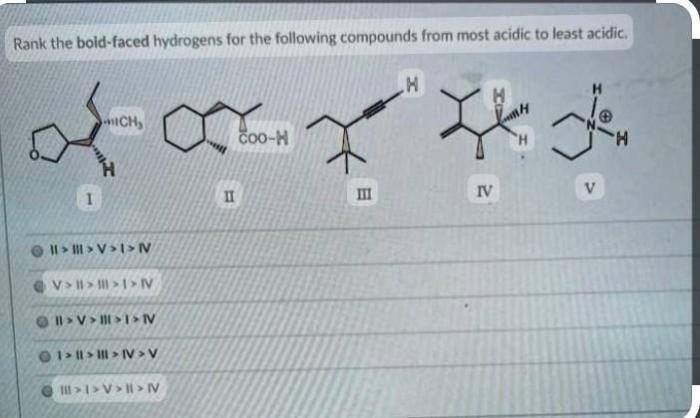
The field of hydrogen ranking is constantly evolving, with new advancements being made all the time. These advancements are driven by the need for more accurate and efficient methods of ranking hydrogens, as well as the development of new applications for hydrogen ranking.
One of the most recent advancements in hydrogen ranking is the development of machine learning methods. Machine learning methods can be used to automatically learn the relationship between the structure of a molecule and the reactivity of its hydrogens. This information can then be used to rank the hydrogens in a molecule, even if the molecule is very large or complex.
Challenges and Opportunities
The development of new hydrogen ranking methods presents both challenges and opportunities. One of the challenges is the need for accurate and reliable data. In order to develop accurate hydrogen ranking methods, it is important to have a large dataset of molecules with known hydrogen reactivity.
This data can be difficult to obtain, as it requires specialized experimental techniques.
Another challenge is the need for efficient hydrogen ranking methods. Hydrogen ranking methods can be computationally expensive, especially for large molecules. This can make it difficult to use hydrogen ranking methods in practical applications.
Despite these challenges, the development of new hydrogen ranking methods also presents a number of opportunities. Hydrogen ranking methods can be used to improve the accuracy of a wide range of chemical predictions. For example, hydrogen ranking methods can be used to predict the reactivity of molecules in chemical reactions, the selectivity of catalysts, and the toxicity of chemicals.
Future of Hydrogen Ranking, Rank the bold faced hydrogens
The future of hydrogen ranking is bright. As new advancements are made, hydrogen ranking methods will become more accurate, efficient, and versatile. This will open up new opportunities for the use of hydrogen ranking in a wide range of applications.
One of the most promising applications of hydrogen ranking is in the design of new drugs. Hydrogen ranking methods can be used to identify the most reactive hydrogens in a molecule, which are the most likely to be involved in chemical reactions.
This information can be used to design drugs that are more effective and have fewer side effects.
Another promising application of hydrogen ranking is in the development of new materials. Hydrogen ranking methods can be used to identify the most reactive hydrogens in a molecule, which are the most likely to be involved in chemical reactions. This information can be used to design materials that are stronger, more durable, and more resistant to corrosion.
The future of hydrogen ranking is full of possibilities. As new advancements are made, hydrogen ranking methods will become more accurate, efficient, and versatile. This will open up new opportunities for the use of hydrogen ranking in a wide range of applications.
Question Bank
What is hydrogen ranking?
Hydrogen ranking is a method used to determine the relative reactivity of hydrogen atoms within a molecule. It considers factors such as the electronegativity of neighboring atoms and the presence of resonance structures.
How is hydrogen ranking used in practice?
Hydrogen ranking finds applications in various fields, including drug design, where it helps predict the reactivity of hydrogen atoms in drug molecules. It is also used in materials science to understand the stability and reactivity of hydrogen-containing materials.
What are the limitations of hydrogen ranking?
While hydrogen ranking provides valuable insights, it is important to note that it is a simplified model and may not always accurately predict the reactivity of hydrogen atoms in complex systems.