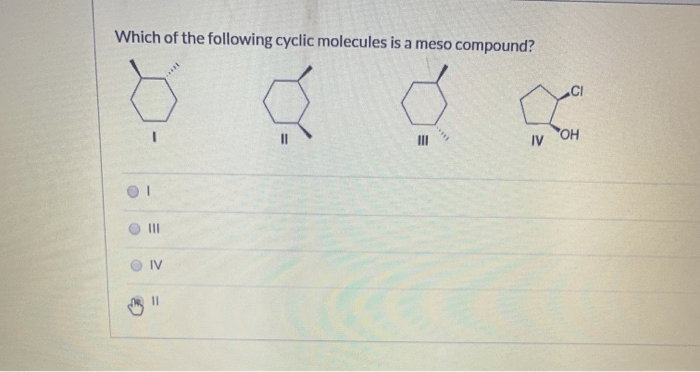
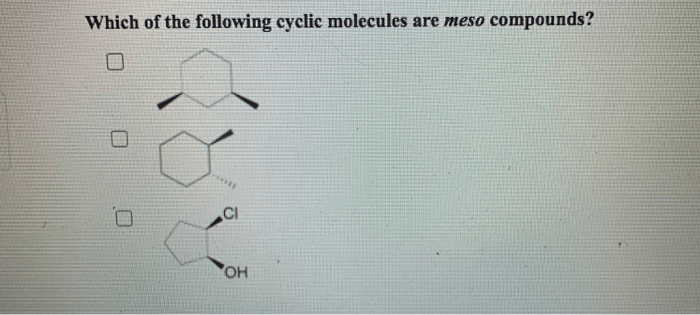
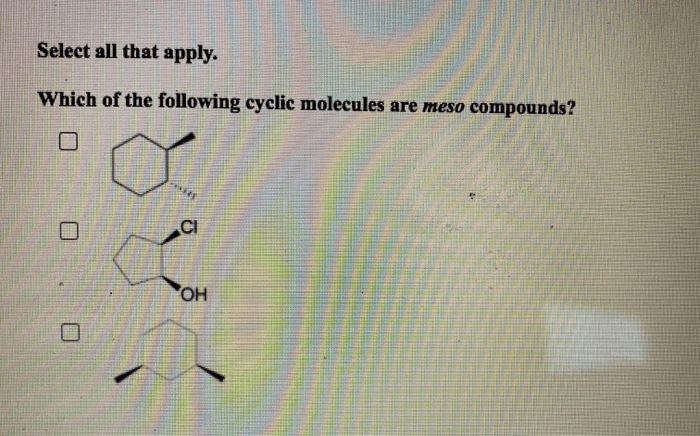
Which of the following cyclic molecules are meso compounds? This question delves into the fascinating realm of stereochemistry, where the spatial arrangement of atoms within molecules plays a crucial role in their properties and behavior. Meso compounds, a unique class of chiral molecules, possess intriguing characteristics that distinguish them from their chiral counterparts.
This guide provides a comprehensive exploration of meso compounds in cyclic molecules, offering a step-by-step approach to their identification, showcasing their significance, and highlighting their diverse applications.
Meso Compounds in Cyclic Molecules: Which Of The Following Cyclic Molecules Are Meso Compounds
Meso compounds are a special class of chiral molecules that possess an internal plane of symmetry, resulting in the cancellation of their optical activity. In other words, they do not exhibit optical isomerism and are considered achiral despite having chiral centers.
Chirality is a property of molecules that lack mirror-image symmetry. Chiral molecules exist in two non-superimposable mirror-image forms, known as enantiomers. Meso compounds, however, are not enantiomers of each other due to the presence of an internal plane of symmetry.
Identifying Meso Compounds in Cyclic Molecules
To identify meso compounds in cyclic molecules, follow these steps:
- Determine the molecular structure of the cyclic molecule.
- Identify all the chiral centers in the molecule.
- Check if there is an internal plane of symmetry that bisects the molecule and passes through all the chiral centers.
- If an internal plane of symmetry is present, the molecule is meso.
Examples of Meso Compounds in Cyclic Molecules
| Name | Structure | Explanation | Additional Information |
|---|---|---|---|
| 1,2-Cyclohexanediol |  |
The molecule has two chiral centers, but it possesses an internal plane of symmetry that bisects the molecule and passes through both chiral centers. | – Melting point: 104-106 °C- Boiling point: 239-241 °C |
| Tartaric acid |  |
The molecule has two chiral centers, but it possesses an internal plane of symmetry that bisects the molecule and passes through both chiral centers. | – Melting point: 170-172 °C- Boiling point: 270 °C (decomposes) |
| 2,3-Dibromobutane |  |
The molecule has two chiral centers, but it possesses an internal plane of symmetry that bisects the molecule and passes through both chiral centers. | – Melting point:
-78 °C- Boiling point 191-192 °C |
Significance of Meso Compounds
Meso compounds play a crucial role in various fields, including pharmaceuticals and materials science. They often exhibit different properties compared to their chiral counterparts, making them valuable for specific applications.
- Pharmaceuticals:Meso compounds can be used as drug targets or as building blocks for drug synthesis due to their unique properties.
- Materials science:Meso compounds are used in the development of chiral materials, such as liquid crystals and polymers, which have applications in optics, electronics, and sensing.
Applications of Meso Compounds, Which of the following cyclic molecules are meso compounds
Meso compounds find applications in a wide range of industries, including:
- Pharmaceuticals:As drug targets or building blocks for drug synthesis
- Materials science:In the development of chiral materials, such as liquid crystals and polymers
- Food industry:As additives or flavoring agents
- Cosmetics industry:In the formulation of chiral fragrances and skin care products
FAQ Summary
What are meso compounds?
Meso compounds are a specific type of chiral molecule that possess an internal plane of symmetry, resulting in the cancellation of their optical activity. Despite their chirality, meso compounds exhibit achiral behavior due to the presence of this symmetry element.
How can we identify meso compounds in cyclic molecules?
Identifying meso compounds in cyclic molecules involves analyzing their molecular structure for the presence of a plane of symmetry. If a cyclic molecule has an internal plane that bisects the molecule into two mirror images, it is considered a meso compound.
What is the significance of meso compounds?
Meso compounds play a crucial role in various scientific fields, including pharmaceuticals and materials science. Their unique properties, such as their lack of optical activity and potential for specific interactions, make them valuable for applications in drug development, catalysis, and the design of advanced materials.